Update on Vaccinations for Dental Healthcare Personnel
Widespread immunization has dramatically altered the global landscape for the transmission of many diseases, reducing morbidity and mortality.1Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bulletin of the World Health Organization 2008;86(2):81-160. Available at: https://www.who.int/bulletin/volumes/86/2/07-040089/en/.,2CDC. Benefits from Immunization During the Vaccines for Children Program Era — United States, 1994–2013. MMWR April 25, 2014;63(16):352-5. General recommendations for childhood and adult vaccinations are designed to minimize the risk of disease transmission among the general public.1Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bulletin of the World Health Organization 2008;86(2):81-160. Available at: https://www.who.int/bulletin/volumes/86/2/07-040089/en/. In addition, immunization is considered an essential component of infection control and prevention in healthcare settings.3CDC. Guidelines for infection control in dental health-care settings — 2003. MMWR Morb Mortal Wkly Rep. 2003;52(RR17);1–61. In the United States, national guidelines on immunizations for healthcare personnel (HCP) are provided by the Advisory Committee on Immunization Practices (ACIP) and the Hospital Infection Control Practices Advisory Committee (HICPAC).4CDC. Immunization of Health-Care Personnel: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011; 60(RR-7). State and federal regulations and recommendations from the Public Health Service and organizations should also be included in policies.3CDC. Guidelines for infection control in dental health-care settings — 2003. MMWR Morb Mortal Wkly Rep. 2003;52(RR17);1–61. The emergence of COVID-19 and the ensuing pandemic have also resulted in the rapid development of vaccines against SARS-CoV-2 and additional recommendations.
Laws and Vaccinations for HCP
Under the Bloodborne Pathogens Standard, the Occupational Safety and Health Administration (OSHA) mandates that all workers at potential risk of exposure to blood or other potentially infectious materials (OPIM) be educated on the risk of transmission, the benefits of vaccination and offered Hepatitis B (HBV) vaccination at no cost and at a reasonable time and place.5OSHA Fact Sheet. Hepatitis B Vaccination Protection. Available at: https://www.osha.gov/OshDoc/data_BloodborneFacts/bbfact05.pdf. Should individuals decline vaccination, they must sign a declination form that must be kept in their personnel file. Following declination, individuals can later request vaccination should they wish to and must then receive this at no cost. State laws for HCP may also mandate vaccinations against some transmissible diseases.6CDC. State Healthcare Worker and Patient Vaccination Laws. Available at: https://www.cdc.gov/phlp/publications/topic/vaccinationlaws.html Mandates vary by State, facility and the role of HCP, making it important to check for your location. In addition, exemptions are granted on medical grounds, and may or may not be permitted on philosophical or religious grounds.7Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association Between Vaccine Refusal and Vaccine-Preventable Diseases in the United States: A Review of Measles and Pertussis. doi: 10.1001/jama.2016.1353.,8Healthcare Training Leader. Carrot or Stick: Immunization Laws for Healthcare Workers, January 14, 2020. Available at: https://healthcare.trainingleader.com/2020/01/immunization-laws-for-healthcare-workers/. Healthcare facilities may also have policies mandating vaccinations, for example, dental schools can mandate vaccinations for students before they begin their curriculum.9Tufts School of Dental Medicine. Immunization & Health Insurance. Immunization Requirements. Available at: https://dental.tufts.edu/immunization-health-insurance. ,10The Ohio State University College of Dentistry. Immunization Requirements. Available at: https://dentistry.osu.edu/dental-hygiene/immunization-requirements. Immunizing students before they are at risk of exposure when treating patients protects students and helps to protect others in the school environment, including patients.
Recommendations for routine vaccination of dental healthcare personnel (DHCP)
Vaccination is recommended for diseases known to represent a substantial risk for transmission in healthcare settings. For DHCP, this has included immunization against HBV, measles, mumps, rubella, varicella, tetanus, pertussis, diphtheria and influenza unless as noted an individual is already immune to a given disease or the vaccine is contraindicated for that individual.3CDC. Guidelines for infection control in dental health-care settings — 2003. MMWR Morb Mortal Wkly Rep. 2003;52(RR17);1–61. (see Table 1 for contraindications for vaccines) Vaccines against COVID-19 have now been added as a new vaccine.
HBV vaccine
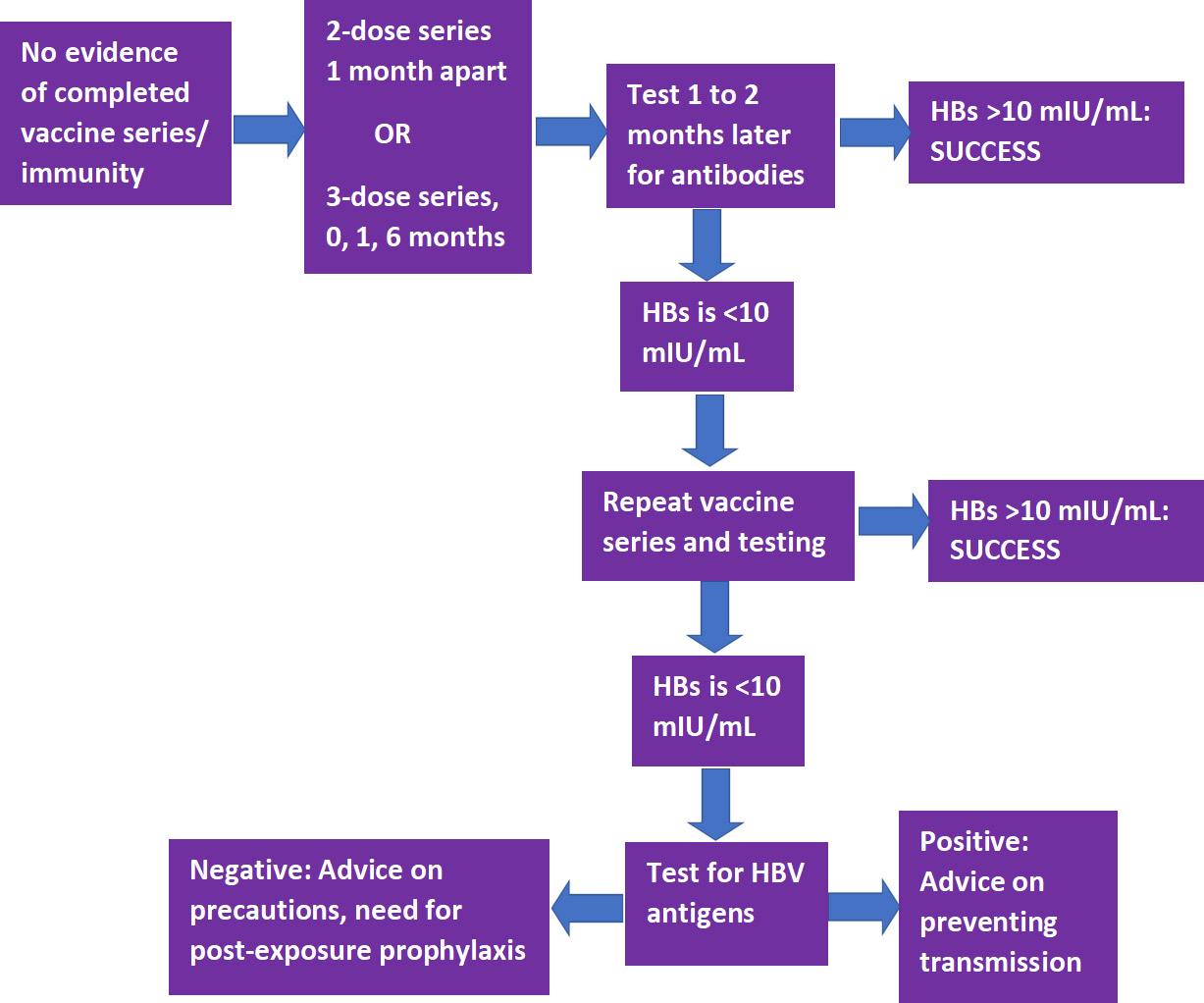
Vaccination against HBV is recommended unless there is documented evidence of a completed vaccine series or there is serologic evidence of immunity.11CDC. Recommended Vaccines for Healthcare Workers. Updated 2016. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. As noted above, this vaccination must be offered to DHCP at risk of exposure to bloodborne pathogens. The CDC Guidelines for infection control in dental health-care settings — 2003, which were published prior to the development of a 2-dose series, recommend vaccination as a 3-dose series to individuals at potential risk of exposure.3CDC. Guidelines for infection control in dental health-care settings — 2003. MMWR Morb Mortal Wkly Rep. 2003;52(RR17);1–61. However, in accordance with the more recent CDC recommendations on vaccinations for HCP, which explicitly includes DHCP and students, HBV vaccination can be given as a 2-dose series with the doses 1 month apart (Heplisav-B) or as a 3-dose series at months 0, 1 and 6 (Engerix-B or Recombivax HB).11CDC. Recommended Vaccines for Healthcare Workers. Updated 2016. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html.
Following completion of a vaccine series, serological testing for Hepatitis B surface antibody (anti-HBs) should be performed 1 to 2 months later.3CDC. Guidelines for infection control in dental health-care settings — 2003. MMWR Morb Mortal Wkly Rep. 2003;52(RR17);1–61.,11CDC. Recommended Vaccines for Healthcare Workers. Updated 2016. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. If the level of anti-HBs is <10 mIU/mL, the individual should receive a second series and repeat serological testing. If testing still indicates an inadequate response, the individual is considered a ‘non-responder.’ Separate testing is then recommended to determine if the individual is positive for HBV antigens. If this test result is positive, advice should be provided on how to prevent transmission to others. If negative, advice should be given on precautions to take to prevent infection, and of the need for post-exposure prophylaxis should a confirmed/probable exposure occur. (Figure 1)
Measles, mumps and rubella (MMR) vaccine
The recommendation for MMR immunization is based on age.11CDC. Recommended Vaccines for Healthcare Workers. Updated 2016. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. For individuals born in 1957 or later, in the absence of prior MMR vaccination or serological evidence of immunity to measles or mumps, a 2-dose series of MMR vaccine with the doses at least 4 weeks apart is recommended (for rubella, a single dose is sufficient).11CDC. Recommended Vaccines for Healthcare Workers. Updated 2016. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. During a mumps outbreak, a third dose of mumps-virus-containing vaccine is recommended for previously vaccinated at-risk individuals.12CDC. Recommendation of the Advisory Committee on Immunization Practices for Use of a Third Dose of Mumps Virus–Containing Vaccine in Persons at Increased Risk for Mumps During an Outbreak. MMWR 2018;67(1);33–38. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6701a7.htm. Individuals born before 1957 are considered immune, while consideration should be given to vaccination for unvaccinated HCP if there is no laboratory evidence of disease or immunity to rubella (1-dose) or measles and/or mumps (2-dose), and during an outbreak of these diseases the respective 1- or 2-dose vaccination schedule is recommended.11CDC. Recommended Vaccines for Healthcare Workers. Updated 2016. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. Women should not receive this vaccine while pregnant and should avoid becoming pregnant for 3 months following vaccination.13National Vaccine Information Center. Who should not get Measles vaccine? Available at: https://www.nvic.org/vaccines-and-diseases/measles/who-should-not-get-measles-vaccine-mmr.aspx.
Tetanus/Diphtheria/Pertussis (Td/Tdap) vaccine
Tdap as a single-dose vaccine is recommended if not previously received/unknown, even if Td was previously given.11CDC. Recommended Vaccines for Healthcare Workers. Updated 2016. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. In addition, a Td/Tdap booster should be given every 10 years. Revaccination is recommended during each pregnancy.
Varicella vaccine
A 2-dose series (doses at least 4 weeks apart) is recommended for unvaccinated DHCP, those who have not had chickenpox/ no serological evidence of immunity.11CDC. Recommended Vaccines for Healthcare Workers. Updated 2016. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. Women should not receive this vaccine while pregnant and should avoid becoming pregnant for 3 months following vaccination.14National Vaccine Information Center. Who should not get Chickenpox vaccine? Available at: https://www.nvic.org/vaccines-and-diseases/chickenpox/vaccine-who-should-not-get.aspx.
Influenza
Annual immunization against influenza is recommended and generally given as inactivated vaccine administered by injection. Live attenuated influenza vaccine (administered nasally) or inactivated vaccine may be given to non-pregnant individuals below the age of 50.11CDC. Recommended Vaccines for Healthcare Workers. Updated 2016. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. In addition, while it is unlikely that dental healthcare personnel would come in close contact with severely immunosuppressed patients requiring protective isolation, should this be the case then inactivated vaccine is preferred.
| Table 1. Recommended vaccines for DHCP | Contraindications | |
|---|---|---|
| Hepatitis B |
|
|
| MMR |
|
|
| Td/Tdap |
|
|
| Varicella |
|
|
| Influenza |
|
|
| COVID-19 |
|
|
COVID-19
SARS-CoV-2 vaccines reviewed by the FDA and granted Emergency Use Authorization (EUA) are now available.18U.S. Food & Drug Administration. FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine. December 11, 2020. Available at: https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19. ,19U.S. Food & Drug Administration. FDA Takes Additional Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for Second COVID-19 Vaccine. Available at: https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid. It is critically important that immunization for as many people as possible occurs to combat the ongoing pandemic. The effectiveness of available vaccines based on mRNA technology (Pfizer/BioNTech and Moderna) is ≥90%, substantially higher than that of annual influenza vaccines, and the results of clinical trials showed a good safety profile. A 2-f series is required, with the doses 21 and 28 days apart, respectively, for the Pfizer/BioNTech and Moderna vaccines.
For the initial roll-out, with a limited vaccine supply, the CDC issued guidance in December 2020 on prioritization for immunization based on a report from ACIP which advised that HCP be among those offered the first doses. ACIP also stated that federal, state and local jurisdictions should use this guidance for COVID-19 vaccination program planning and implementation. There was, however, concern that the advisory could be interpreted to exclude some categories of HCP, such as DHCP, based on different infection control guidelines for these groups.20Coalition Letter, COVID-19 Vaccination Playbook for Jurisdictional Operations. December 16, 2020. Available at: https://www.ada.org/~/media/ADA/Advocacy/Files/201216_cdc_ncird_covid19_coalition.pdf. The CDC recommendations were therefore updated on December 29, 2020 and explicitly include prioritizing DHCP including students, and those other groups in Phase 1.21CDC. The Importance of COVID-19 Vaccination for Healthcare Personnel. Updated December 28, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/hcp.html. ,22https://www.ada.org/en/publications/ada-news/2021-archive/january/cdc-confirms-dentists-in-first-phase-of-covid-19-vaccinations. Each State jurisdiction and county can determine prioritized groups for their vaccination program.
Recommendations and the Role of Dental Professionals
The CDC recommends that all dental settings develop a written health program that addresses immunizations, screening for tuberculosis, work restrictions and other occupational health needs.23CDC. Dental Health Care Personnel Safety and Program Evaluation https://www.cdc.gov/oralhealth/infectioncontrol/summary-infection-prevention-practices/personal-safety-program-evaluation.html. With respect to diseases for which immunization is recommended, DHCP with active measles/mumps/rubella/varicella or who are susceptible to any of these and were exposed are excluded from duty. For pertussis, DHCP are excluded for duty if they have active disease or were exposed and are symptomatic. Details on duty exclusion and its duration can be found in the CDC guidelines and recommendations, with the exception of COVID-19 for which guidance on quarantining was issued.3CDC. Guidelines for infection control in dental health-care settings — 2003. MMWR Morb Mortal Wkly Rep. 2003;52(RR17);1–61.,24ADA. What to Do if Someone on Your Staff Tests Positive for COVID-19. Available at: https://success.ada.org/~/media/CPS/Files/COVID/A_Positive_COVID-19_Test_Result_On_Your_Staff.pdf?la=en. In addition, a comprehensive written policy including a list of all recommended and required immunizations is recommended.
Immunization of DHCP against specific communicable diseases as recommended by the CDC and ACIP reduces host susceptibility and also helps to reduce the potential for transmission to co-workers and patients.3CDC. Guidelines for infection control in dental health-care settings — 2003. MMWR Morb Mortal Wkly Rep. 2003;52(RR17);1–61. Dental professionals can further play a role in disease prevention by educating patients on the benefits of vaccination and by debunking misinformation25Hotez P. America and Europe’s new normal: the return of vaccine-preventable diseases. Pediatr Res 2019;85(7):912-4. doi:10.1038/s41390-019-0354-3.. It is especially important at the current time with respect to COVID-19 vaccines that key information is provided to patients regarding vaccine efficacy and safety to encourage vaccination. In addition, depending on the scope of practice for a given State, dental professionals may be able to administer vaccines.
Scope of Practice
State laws dictate whether the scope of practice permits dental professionals to administer vaccines and, if so, which ones. The first State to permit dentists to immunize patients of all ages with many vaccine types was Oregon, after the Oregon House Bill 2220 was signed on May 6, 2019.26Oregon Health & Science University. Oregon Dental Immunization Resources. Available at: https://www.ohsu.edu/school-of-dentistry/oregon-dental-immunization-resources. The administration of vaccines by dentists is supported by the American Dental Association (ADA).27ADA News. ADA supports efforts allowing dentists to administer vaccines, October 23, 2020. Available at: https://www.ada.org/en/publications/ada-news/2020-archive/october/ada-supports-efforts-allowing-dentists-to-administer-vaccines. A number of States now allow dentists to administer vaccines against COVID-19 during the current emergency.28ADA. COVID-19 Vaccine Regulations for Dentists Map. https://success.ada.org/en/practice-management/patients/covid-19-vaccine-regulations-for-dentists-map. In Nevada, as of January 13, 2021 dentists and dental hygienists may administer COVID-19 vaccines with EUA and the State Board of Dental Examiners passed emergency regulations permitting licensed dental professionals to do so provided they first complete a required certification training program.29Nevada Dental Hygienists’ Association. Not going to miss my shot. Available at: https://nvdha.com/. Information on the ADA website can be found on vaccine allocation and administration by dentists for some States and contains links for further information.28ADA. COVID-19 Vaccine Regulations for Dentists Map. https://success.ada.org/en/practice-management/patients/covid-19-vaccine-regulations-for-dentists-map. Requirements by State vary and may include, for example, additional training. Restrictions on where the vaccine may be administered must also be followed. It is critical to check with your State and State Board on the regulations for dentists and dental hygienists, and to ensure that all regulations and requirements are followed.
Conclusions
Recent events and the EUA of COVID-19 vaccines highlights the importance of immunization. Dental professionals can play a key role in helping to prevent disease transmission by following the recommendations, and educating patients on the benefits, efficacy and safety of vaccinations (in the absence of contraindications). In addition, where permitted and following regulations and recommendations, dental professionals can assist in the implementation of vaccination against COVID-19. Following the CDC recommendations on vaccinations is a key component of infection control and prevention and in protecting DHCP and patients.
References
- 1.American Association of Pediatric Dentistry. Guidelines for the management of traumatic dental injuries: 2. Avulsion of permanent teeth. Reference Manual 2013;39(6):412-9.
- 2.Andersson L, Andreasen JO, Day P, Heithersay G, Trope M, DiAngelis AJ, Kenny DJ, Sigurdsson A, Bourguignon C, Flores MT, Hicks ML, Lenzi AR, Malmgren B, Moule AJ, Tsukiboshi M. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: 2. Avulsion of permanent teeth. Dent Traumatol 2012;28:88-96.
- 3.Bagattoni S, Sadotti A, D’Alessandro G, Piana G. Dental trauma in Italian children and adolescents with special health care needs. A cross-sectional retrospective study. Eur J Paediatr Dent 2017;18:23-6.
- 4.Lee JY, Divaris K. Hidden consequences of dental trauma: The social and psychological effects. Pediatr Dent 2009;31:96-101.
- 5.American Association of Endodontists. The recommended guidelines of the American Association of Endodontists for the treatment of traumatic dental injuries, 2013. Available at: http://www.nxtbook.com/nxtbooks/aae/traumaguidelines/index.php#/4
- 6.Petrovic B, Marković D, Peric T, Blagojevic D. Factors related to treatment and outcomes of avulsed teeth. Dent Traumatol 2010;26(1):52-9. doi: 10.1111/j.1600-9657.2009.00836.x.
- 7.Poi WR, Sonoda CK, Martins CM, Melo ME, Pellizzer EP, de Mendonça MR, Panzarini SR. Storage media for avulsed teeth: a literature review. Braz Dental J 2013;24(5):437-45.
- 8.Andreasen JO, Borum MK, Jacobsen HL, Andreasen FM. Replantation of 400 avulsed permanent incisors. 4. Factors related to periodontal ligament healing. Endod Dent Traumatol 1995;11(2):76-89.
- 9.Tuna EB, Arai K, Tekkesin MS, Seymen F, Gencay K, Kuboyama N, Maeda T. Effect of fibroblast growth factor and enamel matrix derivative treatment on root resorption after delayed replantation. Dent Traumatol 2015;31(1):49-56. doi: 10.1111/edt.12141.
- 10.Andreasen JO, Borum MK, Jacobsen HL, Andreasen FM. Replantation of 400 avulsed permanent incisors. 2. Factors related to pulpal healing. Endod Dent Traumatol 1995;11(2):59-68.
- 11.Andreasen JO, Borum MK, Andreasen FM. Replantation of 400 avulsed permanent incisors. 3. Factors related to root growth. Endod Dent Traumatol 1995;11(2):69-75.
- 12.Is Khinda V, Kaur G, G SB, Kallar S, Khurana H. Clinical and practical implications of storage media used for tooth avulsion. Int J Clin Pediatr Dent 2017;10:158-65.
- 13.Hasan MR, Takebe H, Shalehin N, Obara N, Saito T, Irie K. Effects of tooth storage media on periodontal ligament preservation. Dent Traumatol 2017;33:383-92.
- 14.Kokkali VV, Bendgude V, Sharangpani G. Comparative evaluation of post-traumatic periodontal ligament cell viability using three storage media. Eur Arch Paediatr Dent 2017;18:209-14.
- 15.Saini D, Gadicherla P, Chandra P, Anandakrishna L. Coconut milk and probiotic milk as storage media to maintain periodontal ligament cell viability: an in vitro study. Dent Traumatol 2017;33(3):160-4.
- 16.Lee W, Stover S, Rasoulianboroujeni M, Sherman K, Fahimipour F. The efficacy of commercial tooth storage media for maintaining the viability of human periodontal ligament fibroblasts. Int Endod J 2018;51:58-68.
- 17.de Souza BD, Bortoluzzi EA, Reyes-Carmona J, Dos Santos LG, Simões CM, Felippe WT, Felippe MC. Effect of temperature and seven storage media on human periodontal ligament fibroblast viability. Dent Traumatol 2017;33(2):100-5. doi: 10.1111/edt.12311.
- 18.Ozer S, Yilmaz E, Bayrak S, Tunc ES. Parental knowledge and attitudes regarding the emergency treatment of avulsed permanent teeth. Eur J Dent 2012;6(4):370-5.
- 19.Tiano L, Barbiero S, Avato FA, Bergmann M, Gaudio RM. Management of facial trauma with tooth avulsion in children and adolescent, investigation on knowledge of parents and teachers. Eur J Pub Health 2015;25(Suppl 3): October 2015. https://doi.org/10.1093/eurpub/ckv176.241
- 20.Phelps JLB. Virginia emergency department physician knowledge of the emergent treatment of avulsed teeth. Thesis, Virginia Commonwealth University, 2008. Available at: https://scholarscompass.vcu.edu/cgi/viewcontent.cgi?article=1844&context=etd.
- 21.Kotha A, Kumar YV, Prathima V, Pratibha B, Ankitha CH. Evaluation of awareness of first aid of avulsed tooth among physicians and nurses of hospital emergency departments: A short survey. Int J Comm Health Med Res 2017;3(3):74-7.
- 22.Baginska J, Rodakowska E Milewski R, Wilczynska-Borawska M, Kierklo A. Polish school nurses’ knowledge of the first-aid in tooth avulsion of permanent teeth. BMC Oral Health 2016;16:30.
- 23.Abu-Dawoud M, Al-Enezi R, Andersson L. Knowledge of emergency management of avulsed teeth among young physicians and dentist. Dental Traumatol 2008;23(6):348-55.
- 24.Raibrown A, Giblin LJ, Boyd LD, Perry K. Gastroesophageal Reflux Disease Symptom Screening in a Dental Setting. J Dent Hyg 2017;91:44-8.
- 25.Drugs.com H2 antagonists. https://www.drugs.com/drug-class/h2-antagonists.html.
- 26.Drugs.com. Protein pump inhibitors. https://www.drugs.com/drug-class/proton-pump-inhibitors.html
- 27.Drugs.com. Sucralfate. https://www.drugs.com/mtm/sucralfate.html.
- 28.Magalhães AC, Wiegand A, Rios D, Buzalaf MAR, Lussi A. Fluoride in dental erosion. Monogr Oral Sci 2011;22:158-70. doi: 10.1159/000325167.
- 29.Teixeira L, Manarte-Monteiro P, Manso MC. Enamel lesions: Meta-analysis on effect of prophylactic/therapeutic agents in erosive tissue loss. J Dent Sci 2016;11(3):215-24. doi: 10.1016/j.jds.2016.03.008.
- 30.Hines D, Xu S, Stranick M, Lavender S, Pilch S, Zhang YP, et al. Effect of a stannous fluoride toothpaste on dentinal hypersensitivity: In vitro and clinical evaluation. J Am Dent Assoc 2019;150(4S):S47-S59. doi: JADA article
- 31.American Dental Association. Erosive tooth wear. https://www.ada.org/en/member-center/oral-health-topics/erosive-tooth-wear.
- 32.Scully CBE C. Drug effects on salivary glands: dry mouth. Oral Dis 2003;9:165-76. doi:10.1034/j.1601-0825.2003.03967.x
- 33.Tang KD, Vasani S, Taheri T, Walsh LJ, Hughes BGM, Kenny L, Punyadeera C. An occult HPV-driven oropharyngeal squamous cell carcinoma discovered through a saliva test. Front Oncol 2020;10:408. doi: 10.3389/fonc.2020.00408.
- 34.OralDNA. OraRisk HPV. https://www.oraldna.com/test/ohpv-complete/
- 35.Wang Y, Springer S, Mulvey CL, Silliman N, Schaefer J, Sausen M, et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med 2015;7:293ra104. doi: 10.1126/scitranslmed.aaa8507.
- 36.Zielińska K, Karczmarek-Borowska B, Kwaśniak K, Czarnik-Kwaśniak J, Ludwin A, Lewandowski B, Tabarkiewicz J. Salivary IL-17A, IL-17F, and TNF-α are associated with disease advancement in patients with oral and oropharyngeal cancer. J Immunol Res 2020:3928504. doi: 10.1155/2020/3928504.
- 37.Lee LT, Wong YK, Hsiao HY, Wang YW, Chan MY, Chang KW. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int J Oral Maxillofac Surg 2018;47(6):699-707. doi: 10.1016/j.ijom.2017.09.016.
- 38.Shen S, Saito Y, Ren S, Liu C, Guo T, Qualliotine J, et al. Targeting viral DNA and promoter hypermethylation in salivary rinses for recurrent HPV-positive oropharyngeal cancer. Otolaryngol Head Neck Surg 2020;162(4):512-9. doi: 10.1177/0194599820903031.
- 39.Hanna GJ, Lau CJ, Mahmood U, Supplee JG, Mogili AR, Haddad RI, et al. Salivary HPV DNA informs locoregional disease status in advanced HPV-associated oropharyngeal cancer. Oral Oncol 2019;95:120-6. doi: 10.1016/j.oraloncology.
- 40.Malamud D. Saliva as a diagnostic fluid. Dent Clin North Am 2011;55(1):159-78. doi:10.1016/j.cden.2010.08.004.
- 41.Collins FM. Integrating the 4Ps into patient care. Available at: https://www.colgateoralhealthnetwork.com/article/integrating-the-4ps-into-patient-care/.
- 42.Knight ET, Murray Thomson W. A public health perspective on personalized periodontics. Periodontol 2000. 2018 Oct;78(1):195-200. doi: 10.1111/prd.12228. PMID: 30198135.
- 43.Picciani BLS, Domingos TA, Teixeira-Souza T et al. Evaluation of the Th17 pathway in psoriasis and geographic tongue. An Bras Dermatol 2019;94(6):677-83. doi: 10.1016/j.abd.2019.01.006.
- 44.National Psoriasis Foundation. Soriatane (Acitretin). https://www.psoriasis.org/soriatane-acitretin/.
- 45.National Psoriasis Foundation. Current Biologics on the Market. https://www.psoriasis.org/current-biologics-on-the-market/.
- 46.Dalmády S, Kemény L, Antal M, Gyulai R. Periodontitis: a newly identified comorbidity in psoriasis and psoriatic arthritis. Expert Rev Clin Immunol 2020;16(1):101-8. doi: 10.1080/1744666X.2019.1700113.


:sharpen(level=0):output(format=jpeg)/up/2023/05/Fiona-Collins-thumbnail-1-3.jpg)
:sharpen(level=0):output(format=jpeg)/up/2021/02/update-on-vaccinations-dhp-2.jpg)